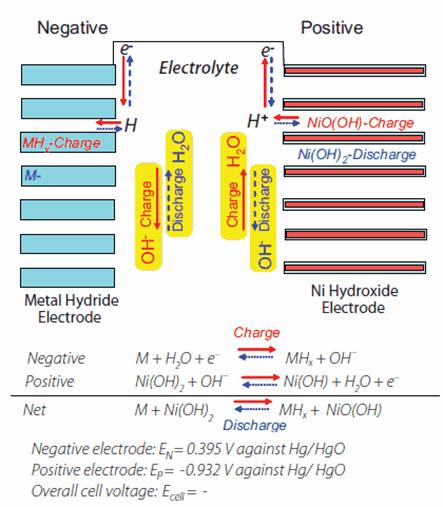
Scheme of Ni-MH-battery below
Nickel–metal hydride battery
Nickel-metal hydride batteries (Ni-MH) – secondary galvanic cell in which the anode is a metal hydride hydrogen electrode (usually a nickel hydride or nickel-lanthanum-lithium), an electrolyte – potassium hydroxide cathode – nickel oxide. New metal hydride compound sufficiently stable for use in batteries, developed in 1980 year. Since the late 1980s, the NiMH batteries are constantly improved, mainly on the stored energy density. Battery Parameters: theoretical energy capacity of 300 Wh/kg, the specific power consumption of about 60-72 Wh/kg, the specific energy density of about 150 Wh/l, emf 1.25 V, a life of about 300-500 charge/discharge cycles , self-discharge up to 100% per year (the old battery types). In the nickel-metal hydride battery "Krona" type, as a rule – the initial voltage of 8.4 V, the voltage gradually reduced to 7.2 V, and then, when the battery power is exhausted, the voltage decreases rapidly. This type of battery is designed to replace the nickel-cadmium batteries. Nickel-metal hydride batteries are approximately 20% more capacity with the same dimensions, but a shorter life of 200 to 300 charge/discharge cycles. Self-discharge is about 1.5-2 times higher than that of nickel-cadmium batteries.
Nickel-metal hydride batteries with a low self-discharge (the low self-discharge nickel-metal hydride battery, LSD NiMH), were introduced by Sanyo in November 2005 under the brand Eneloop brand. This type of battery has a reduced self-discharge, and therefore has a longer shelf life as compared to conventional NiMH. Batteries are sold as "ready to use" or "pre-charged" and positioned as a replacement for alkaline batteries. Nickel-metal hydride batteries with a low self-discharge typically have much lower internal resistance than conventional batteries, the NiMH. It affects very positively in devices with high current consumption: a more stable voltage, reduced heat dissipation, especially in the rapid charge/discharge mode, higher efficiency, the ability to high pulsed current output (example: camera flash is charging is faster), the possibility of continuous operation in devices low power consumption (example: remote controls, watches).
Applications: replacement of the standard electrochemical cell electric vehicles, defibrillators, rocket and space technology, independent power supply system, radio equipment, lighting equipment.
Ni-MH-battery design. Positive electrode: nickel oxide NiOOH. Negative electrode: metal alloy (M), which can reversibly absorb hydrogen (forming hydride MH) and desorb it (examples of alloys: LaNi5; TiFe; Mg2Ni). Electrolyte: 26-31% aqueous KOH soluion. Electrochemical system: (-) MH | KOH | NiOOH (+).
Electrochemical processes.The electrode reaction at the positive nickel oxide: Ni(OH)2 + OH- NiOOH + H2O + e- (charge), NiOOH + H2O + e- Ni(OH)2 + OH- (discharge) (E0 = 0.49 B ). The electrode reaction at the negative electrode metal having absorbed hydrogen is converted into metal hydride: M + H2O + e- MH + OH- (charge), MH + OH- M + H2O + e- (discharge) (E0»-0.9 B). The general reaction in Ni-MH battery is recorded as follows: Ni(OH)2 + M NiOOH + MH (charge), NiOOH + MH Ni(OH)2 + M (discharge).
Scheme of Ni-MH-battery below