Describe the main types of alkaline maganese-zinc cells.
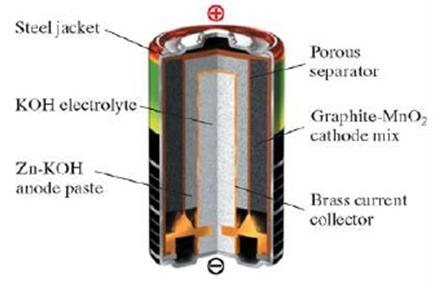
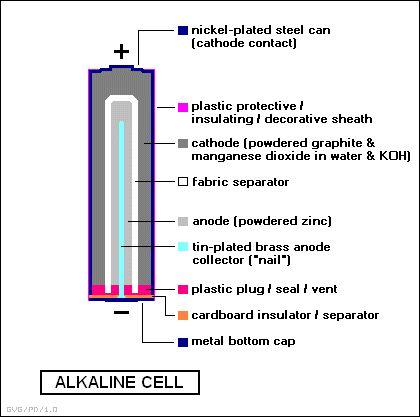
Work on the improvement of consumer properties of primary power sources have resulted in the sixties to the beginning of the production of alkaline batteries. The name of this type of battery received by the electrolyte substance - concentrated alkaline solution. Today, alkaline batteries are often called alkaline due to the inscriptions on the battery housing, issued abroad "Alkaline" (alkaline). The use as an electrolyte salt instead of alkali solution can dramatically improve the performance characteristics of batteries. During the reaction, the electrolyte is consumed very little, so it takes less than a salt battery. The negative electrode is a zinc powder, which occupies 20-30% of the volume, rather than a glass like salt batteries. Battery design makes it possible to significantly increase the service life and increase the maximum current, to give to the load. A negative electrode disposed in the central portion of the batteries is a paste of zinc powder and electrolyte thickener. Use of the powder can significantly increase the area of contact of the electrode with the electrolyte, which reduces the internal resistance of the battery and the discharge current increases. A porous membrane impregnated with electrolyte separates the negative and positive electrodes. The positive electrode comprises a mixture of manganese oxide and graphite, which fills the volume between the membrane and sealed steel casing batteries. The lack of separation of gases in electrochemical reaction in the battery allows you to take her body tight. At the bottom of the battery is a safety valve to protect the battery from the explosion.
Manganese-zinc alkaline batteries are used as a separate independent element equipment supply. Repair weaknesses manganese-zinc salt batteries in the manganese-zinc alkaline. They increase the capacity, enhanced tightness, increased strength gaskets. By reducing the internal resistance of the battery discharge current is increased. Manganese-zinc cells and batteries are designed to power the radio and measuring devices, low-power motors and for lighting. Therefore, they must be capable of operating at continuous long and short discharge mode, as well as intermittent operation.
The oxidizing agent is manganese dioxide, and the reducing agent - in the form of zinc powder, which significantly develop surface and thereby reduce the passivation of the zinc surface during high current discharge. Cathode: Electrolytic manganese oxide MnO2, graphite mixed with an alkali solution and binders. Anode: paste of 30% strength potassium hydroxide solution, KOH, gelled starch, wherein the zinc powder is distributed. Electrolyte: KOH (27-40% or 6/10 mol/l); NaOH (20% or 6 mol/l).
Zinc electrode in an alkaline electrolyte: primary process. Medium and large current density Zn+4OH-ZnO22-+2H2O+2e- (Ea=-1.216 В). Features: consumes a large amount of alkali; formed zincate soluble (solubility of 1-2 mol/l); at saturation zincate solution is deposited on the surface of zinc Zn(OH)2 - a primary process is terminated; therefore, the capacity of the zinc electrode in this case is not limited by the amount of zinc, and the amount of alkali. Zinc electrode in an alkaline electrolyte: secondary process. Small current density: Zn + 2OH- Zn(OH)2 + 2e-, or Zn + 2OH- ZnO + H2O + 2e- (Ea=-1.245 V). Features: alkali consumption in 2 times less than in the primary process; form insoluble hydroxide or oxide; capacity of the zinc electrode in this case is not limited by the amount of alkali at the cathode: for a one electron is released ion OH-.
Powdered zinc electrode provides a substantial increase in active material utilization as compared with salt elements. With uninterrupted discharge medium and high currents, alkaline batteries provide high capacity (up to 7-10 times) than saline elements of the same size. The rate of self-discharge alkaline manganese-zinc elements less: After 1 year of storage at +20°C or 3 months at +50°C, the loss of capacity is approximately 10% of the initial capacity.