Scientific Method Definition
Atomic Structure
Atoms are in your body, the chair you are sitting in, your desk and even in the air.
Before we talk about the physics of atomic bombs, it's a good idea to go over the basic properties of atoms.
Atoms are incredibly small -- the smallest is about 10-8 cm in diameter. For an idea of how small this really is, think of a baseball. The diameter of a baseball is about 7 cm. If an atom were the size of a baseball, an actual baseball would be about 3044 miles high.
An atom is made up of three subatomic particles -- protons, neutrons and electrons. The center of an atom, called the nucleus, is composed of protons and neutrons. Protons are positively charged, neutrons have no charge at all and electrons are negatively charged. The proton-to-electron ratio is always one to one, so the atom as a whole has a neutral charge. For example, a carbon atom has six protons and six electrons.
An atom's properties can change considerably based on how many of each particle it has:
· The number of protons in an atom determines the type of element. Elements are classified by their atomic number, which is simply the number of protons in an atom's nucleus. Some common elements on Earth are oxygen, carbon and hydrogen. You can see the elements on the periodic table here.
· There are different types of atoms called isotopes. These isotopes look and act the same in nature -- the only difference is the number of neutrons in the nucleus.
· You can calculate the “mass” of an atom by counting the number of protons and neutrons inside the nucleus. This number is called the atomic mass. Carbon has three isotopes, for example -- carbon-12 (six protons + six neutrons), carbon-13 (six protons + seven neutrons) and carbon-14 (six protons + eight neutrons).
Nuclear Energy
Two important concepts in physics explain how massive amounts of energy can come from very small particles -- Einstein's famous equation E = MC2 and nuclear radiation.
E = mc2
An atom's nucleus and the structure of certain isotopes make it possible to release incredible amounts of energy when the atom splits. You can understand how much energy this process releases by looking at Einstein's equation E = mc2, where E is energy, m is mass and c is the speed of light (approximately 300,000 kilometers per second). Although you may have heard of this equation without knowing what it really means, the concept behind it is pretty simple. Matter and energy are essentially interchangeable -- matter can be converted into energy, and energy can be converted into matter, and the numbers involved are enormous. The speed of light is a huge number -- if you multiply a large amount of mass by the speed of light, you get an extreme amount of energy. And even though atoms are small -- they don't have a lot of mass -- it takes a vast number of them to make a substance.
Substances like uranium, which are commonly used in nuclear bombs, have a very high atomic number -- the atoms themselves are larger and contain more particles than the atoms of other naturally-occurring substances. Because of this additional nuclear material, uranium has the power to release a lot of energy. If you multiplied 7 kilograms of uranium by the speed of light squared, you would get about 2.1 billion Joules of energy. By comparison, a 60-watt light bulb uses 60 joules of energy per second. The energy found in a pound of highly enriched uranium is equal to something on the order of a million gallons of gasoline. When you consider that a pound of uranium is smaller than a baseball and a million gallons of gasoline would fill a cube that is 50 feet per side (50 feet is as tall as a five-story building), you can get an idea of the amount of energy available in just a little bit of U-235.
Radioactive decay
Radioactive decay involves atoms splitting or shedding their parts, and these parts leave the atom at high speeds, becoming rays. There are three types of radioactive decay:
· Alpha decay: A nucleus ejects two protons and two neutrons bound together, known as an alpha particle.
· Beta decay: A neutron becomes a proton, an electron and an antineutrino. The ejected electron is a beta particle.
· Spontaneous fission: A nucleus splits into two pieces. In the process, it can eject neutrons, which can become neutron rays. The nucleus can also emit a burst of electromagnetic energy known as a gamma ray. Gamma rays are the only type of nuclear radiation that comes from energy instead of fast-moving particles.
Scientific Method Definition
Science is practical. Although science sometimes involves learning from textbooks or professors in lecture halls, its primary activity is discovery. Discovery is an active, hands-on process, not something done by scholars isolated from the world in ivory towers. It is both a search for information and a quest to explain how information fits together in meaningful ways. And it almost always seeks answers to very practical questions: How does human activity affect global warming? Why are honeybee populations suddenly declining in North America? What enables birds to migrate such long distances? How do black holes form?
Science is based on observation. Scientists use all of their senses to gather information about the world around them. Sometimes they gather this information directly, with no intervening tool or apparatus. Other times they use a piece of equipment, such as a telescope or microscope, to gather information indirectly. Either way, scientists will write down what they see, hear and feel. These recorded observations are called data.
Data can reveal the structure of something. This is quantitative data, which describes an object numerically. The following are examples of quantitative data:
· The body temperature of a ruby-throated hummingbird is 40.5°C (105°F).
· The speed of light is 299,792,458 meters per second (670,635,729 mph).
· The diameter of Jupiter is 142,984 kilometers (88,846 miles).
· The length of a blue whale is 30.5 meters (100 feet).
Notice that quantitative data consist of a number followed by a unit. The unit is a standardized way to measure a certain dimension or quantity. For example, the foot is a unit of length. So is the meter. In science, the International System (SI) of units, the modern form of the metric system, is the global standard.
Data can also reveal behavior. This is qualitative data, which are written descriptions about an object or organism. John James Audubon, the 19th-century naturalist, ornithologist and painter, is famous for the qualitative observations he made about bird behavior, such as this one:
Generally, scientists collect both quantitative and qualitative data, which contribute equally to the body of knowledge associated with a certain topic. In other words, quantitative data is not more important or more valuable because it is based on precise measurements .
Cell Theory
The discovery of the cell was made possible by the invention of the microscope, which was made possible by improved lens-grinding techniques. Antoni van Leeuwenhoek (1632-1723), a Dutch tradesman, learned to grind lenses and assemble them into simple microscopes. His contemporary Robert Hooke (1635-1703) used such an instrument to observe cork cells, sketches of which appeared in his 1665 publication "Micrographia." Inspired by Hooke's work, Leeuwenhoek began making microscopic examinations of his own. In 1678, he reported to the Royal Society that he had discovered "little animals" -- bacteria and protozoa -- in various samples. The society asked Hooke to confirm Leeuwenhoek's findings, and he did. This paved the way for wide acceptance that a hidden world existed just beyond the limits of human vision and encouraged many scientists to take up the microscope in their investigations. One such scientist was German botanistMatthias Jakob Schleiden (1804-1881), who looked at numerous plant samples. Schleiden was the first to recognize that all plants, and all the different parts of plants, are composed of cells. While having dinner with zoologist Theodor Schwann (1810-1882), Schleiden mentioned his idea. Schwann, who came to similar conclusions while studying animal tissues, quickly saw the implications of their work. In 1839, he published "Microscopic Investigations on the Accordance in the Structure and Growth of Plants and Animals," which included the first statement of the cell theory: All living things are made up of cells.
Then, in 1858, Rudolf Virchow (1821-1902) extended the work of Schleiden and Schwann by proposing that all living cells must rise from pre-existing cells. This was a radical idea at the time because most people, scientists included, believed that nonliving matter could spontaneously generate living tissue. The inexplicable appearance of maggots on a piece of meat was often given as evidence to support the concept of spontaneous generation. But a famous scientist by the name of Louis Pasteur (1822-1895) set out to disprove spontaneous generation with a now-classic experiment that both firmly established the cell theory beyond doubt and solidified the basic steps of the modern scientific method.
How Car Engines Work
Have you ever opened the hood of your car and wondered what was going on in there? A car engine can look like a big confusing jumble of metal, tubes and wires to the uninitiated. You might want to know what's going on simply out of curiosity. Or perhaps you are buying a new car, and you hear things like "3.0 liter V-6" and "dual overhead cams" and "tuned port fuel injection." What does all of that mean?
In this article, we'll discuss the basic idea behind an engine and then go into detail about how all the pieces fit together, what can go wrong and how to increase performance.
The purpose of a gasoline car engine is to convert gasoline into motion so that your car can move. Currently the easiest way to create motion from gasoline is to burn the gasoline inside an engine. Therefore, a car engine is an internal combustion engine -- combustion takes place internally.
Two things to note:
· There are different kinds of internal combustion engines. Diesel engines are one form and gas turbine engines are another. See also the articles on HEMI engines, rotary engines and two-stroke engines. Each has its own advantages and disadvantages.
· There is such a thing as an external combustion engine. A steam engine in old-fashioned trains and steam boats is the best example of an external combustion engine. The fuel (coal, wood, oil, whatever) in a steam engine burns outside the engine to create steam, and the steam creates motion inside the engine. Internal combustion is a lot more efficient (takes less fuel per mile) than external combustion, plus an internal combustion engine is a lot smaller than an equivalent external combustion engine. This explains why we don't see any cars from Ford and GM using steam engines.
Internal Combustion
The principle behind any reciprocating internal combustion engine: If you put a tiny amount of high-energy fuel (like gasoline) in a small, enclosed space and ignite it, an incredible amount of energy is released in the form of expanding gas. You can use that energy to propel a potato 500 feet. In this case, the energy is translated into potato motion. You can also use it for more interesting purposes. For example, if you can create a cycle that allows you to set off explosions like this hundreds of times per minute, and if you can harness that energy in a useful way, what you have is the core of a car engine!
|
Figure 1
Almost all cars currently use what is called a four-stroke combustion cycle to convert gasoline into motion. The four-stroke approach is also known as the Otto cycle, in honor of Nikolaus Otto, who invented it in 1867. The four strokes are illustrated in Figure 1. They are:
· Intake stroke
· Compression stroke
· Combustion stroke
· Exhaust stroke
You can see in the figure that a device called a piston replaces the potato in the potato cannon. The piston is connected to the crankshaft by a connecting rod. As the crankshaft revolves, it has the effect of "resetting the cannon." Here's what happens as the engine goes through its cycle: 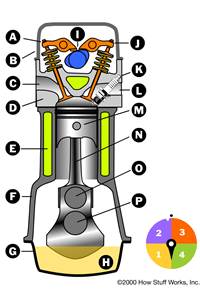 Your browser does not support JavaScript or it is disabled.
Your browser does not support JavaScript or it is disabled.
1. The piston starts at the top, the intake valve opens, and the piston moves down to let the engine take in a cylinder-full of air and gasoline. This is the intake stroke. Only the tiniest drop of gasoline needs to be mixed into the air for this to work. (Part 1 of the figure)
2. Then the piston moves back up to compress this fuel/air mixture. Compression makes the explosion more powerful. (Part 2 of the figure)
3. When the piston reaches the top of its stroke, the spark plug emits a spark to ignite the gasoline. The gasoline charge in the cylinder explodes, driving the piston down. (Part 3 of the figure)
4. Once the piston hits the bottom of its stroke, the exhaust valve opens and the exhaust leaves the cylinder to go out the tailpipe. (Part 4 of the figure)
Now the engine is ready for the next cycle, so it intakes another charge of air and gas.
Notice that the motion that comes out of an internal combustion engine is rotational, while the motion produced by a potato cannon is linear (straight line). In an engine the linear motion of the pistons is converted into rotational motion by the crankshaft. The rotational motion is nice because we plan to turn (rotate) the car's wheels with it anyway.
Basic Engine Parts
The core of the engine is the cylinder, with the piston moving up and down inside the cylinder. The engine described above has one cylinder. That is typical of most lawn mowers, but most cars have more than one cylinder (four, six and eight cylinders are common). In a multi-cylinder engine, the cylinders usually are arranged in one of three ways: inline, V or flat (also known as horizontally opposed or boxer), as shown in the following figures.
Different configurations have different advantages and disadvantages in terms of smoothness, manufacturing cost and shape characteristics. These advantages and disadvantages make them more suitable for certain vehicles.
Let's look at some key engine parts in more detail.
Spark plug
The spark plug supplies the spark that ignites the air/fuel mixture so that combustion can occur. The spark must happen at just the right moment for things to work properly.
Valves
The intake and exhaust valves open at the proper time to let in air and fuel and to let out exhaust. Note that both valves are closed during compression and combustion so that the combustion chamber is sealed.
Piston
A piston is a cylindrical piece of metal that moves up and down inside the cylinder.
Piston rings
Piston rings provide a sliding seal between the outer edge of the piston and the inner edge of the cylinder. The rings serve two purposes:
· They prevent the fuel/air mixture and exhaust in the combustion chamber from leaking into the sump during compression and combustion.
· They keep oil in the sump from leaking into the combustion area, where it would be burned and lost.
Most cars that "burn oil" and have to have a quart added every 1,000 miles are burning it because the engine is old and the rings no longer seal things properly.
Connecting rod
The connecting rod connects the piston to the crankshaft. It can rotate at both ends so that its angle can change as the piston moves and the crankshaft rotates.
Crankshaft
The crankshaft turns the piston's up and down motion into circular motion just like a crank on a jack-in-the-box does.
Sump
The sump surrounds the crankshaft. It contains some amount of oil, which collects in the bottom of the sump (the oil pan).
Engine Problems
So you go out one morning and your engine will turn over but it won't start... What could be wrong? Now that you know how an engine works, you can understand the basic things that can keep an engine from running. Three fundamental things can happen: a bad fuel mix, lack of compression or lack of spark. Beyond that, thousands of minor things can create problems, but these are the "big three." Based on the simple engine we have been discussing, here is a quick rundown on how these problems affect your engine:
Bad fuel mix - A bad fuel mix can occur in several ways:
· You are out of gas, so the engine is getting air but no fuel.
· The air intake might be clogged, so there is fuel but not enough air.
· The fuel system might be supplying too much or too little fuel to the mix, meaning that combustion does not occur properly.
· There might be an impurity in the fuel (like water in your gas tank) that makes the fuel not burn.
Lack of compression - If the charge of air and fuel cannot be compressed properly, the combustion process will not work like it should. Lack of compression might occur for these reasons:
· Your piston rings are worn (allowing air/fuel to leak past the piston during compression).
· The intake or exhaust valves are not sealing properly, again allowing a leak during compression.
· There is a hole in the cylinder.
The most common "hole" in a cylinder occurs where the top of the cylinder (holding the valves and spark plug and also known as the cylinder head) attaches to the cylinder itself. Generally, the cylinder and the cylinder head bolt together with a thin gasket pressed between them to ensure a good seal. If the gasket breaks down, small holes develop between the cylinder and the cylinder head, and these holes cause leaks.
Doing regular engine maintenance can help you avoid future repairs.
Lack of spark - The spark might be nonexistent or weak for a number of reasons:
· If your spark plug or the wire leading to it is worn out, the spark will be weak.
· If the wire is cut or missing, or if the system that sends a spark down the wire is not working properly, there will be no spark.
· If the spark occurs either too early or too late in the cycle (i.e. if the ignition timing is off), the fuel will not ignite at the right time, and this can cause all sorts of problems.
Many other things can go wrong. For example:
· If the battery is dead, you cannot turn over the engine to start it.
· If the bearings that allow the crankshaft to turn freely are worn out, the crankshaft cannot turn so the engine cannot run.
· If the valves do not open and close at the right time or at all, air cannot get in and exhaust cannot get out, so the engine cannot run.
· If someone sticks a potato up your tailpipe, exhaust cannot exit the cylinder so the engine will not run.
· If you run out of oil, the piston cannot move up and down freely in the cylinder, and the engine will seize.
In a properly running engine, all of these factors are within tolerance.
As you can see, an engine has a number of systems that help it do its job of converting fuel into motion. We'll look at the different subsystems used in engines in the next few sections.