Describe the main types of primary electrochemical cells.
A primary cell is a battery that is designed to be used once and discarded, and not recharged with electricity and reused like a secondary cell (rechargeable battery). In general, the electrochemical reaction occurring in the cell is not reversible, rendering the cell unrechargeable. As a primary cell is used, chemical reactions in the battery use up the chemicals that generate the power; when they are gone, the battery stops producing electricity and is useless. In contrast, in a secondary cell, the reaction can be reversed by running a current into the cell with a battery charger to recharge it, regenerating the chemical reactants.
There are a lot of types of primary cells: Manganese-zinc power sources with salt electrolyte (e-t Leklanshe); Manganese-zinc power sources with an alkaline electrolyte; Mercury-zinc power sources; Mercury-cadmium power sources; Silver-zinc primary battery; Copper-zinc power sources; Zinc air primary battery; Lithium primary current sources with a solid cathode and an aprotic electrolyte; Lithium current sources with a liquid or dissolved oxidizing agent; Iodine, lithium power sources with a solid electrolyte.
The main types of primary electrochemical cells: Manganese-zinc power sources with salt electrolyte. The anode is zinc, which is the body of the current source, the cathode active material is the electrolytic manganese dioxide or chemical manganese dioxide, electrolyte is ammonium chloride, zinc chloride, ammonium chloride or zinc chloride. The electrolyte is either viscosified state or in the pores of the microporous separator. To reduce the speed or to prevent corrosion in zinc and the electrolyte is added corrosion inhibitors. The advantages of these batteries are low cost, the disadvantages are the falling discharge curve, relatively low specific energy, a significant deterioration in performance at high loads and low temperatures.
Reaction on anode: Zn (solid) = Zn2+ (aqueous) + 2e. Reaction on cathode: 2NH4+ (aqueous) + 2MnO2 (solid) + 2e = Mn2O3 (solid) + 2NH3 (aqueous) + H2O (liquid). When using ammonium chloride general equation: 2MnO2 + 2NH4Cl + Zn ZnCl2· 2NH3 + H2O + Mn2O3. When using zinc chloride equation: 8MnO2 + 4Zn + 2ZnCl2 + 9H2O 8MnOOH + ZnCl2 · 4ZnO · 5H2O.
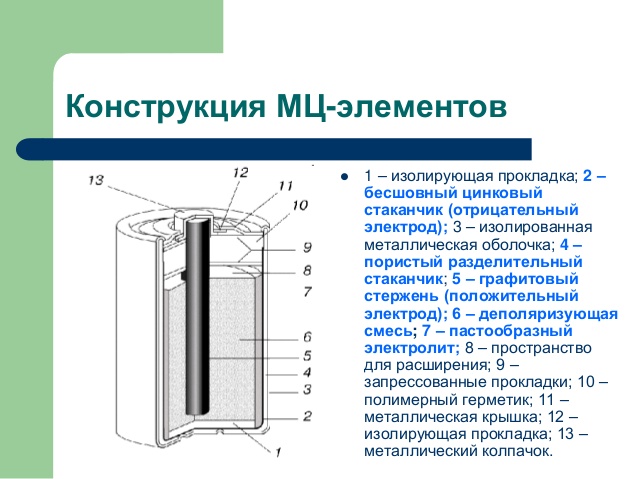
Manganese-zinc power sources with an alkaline electrolyte. The anode is a zinc powder and a cathode is manganese dioxide. The electrolyte is a gel-like solution of KOH or KOH in a matrix. The anode and electrolyte composition include corrosion inhibitors. In comparison with the manganese-zinc-current source with a saline electrolyte battery with an alkaline electrolyte have higher capacity and energy density, especially at high loads and low temperature, but they are more expensive.
Lithium primary current sources with a solid cathode and an aprotic electrolyte. The reducing agent is lithium oxidants - oxides, metal sulfides or fluorocarbon. Electrolytes are the solution of lithium salt (LiClO4, LiBF4 or LiBr) in aprotic solvents: Propylene carbonate (PC), dioxolane (DOL), -butyrolactone (BL), tetrahydrofuran (THF), dimethoxyethane (DME), etc. Depending on the type of oxidant power source has a discharge voltage of about 1.5V.