How is a nuclear equation different from a chemical equation?
TRANSMUTATION
An atomic nucleus can undergo three kinds of nuclear change. These nuclear changes are transmutation, fission, and fusion. In a transmutation,the nuclei of a radioactive element decay at a fixed rate. They do this by emitting nuclear particles. Inthis process, one element is changed into a different element.
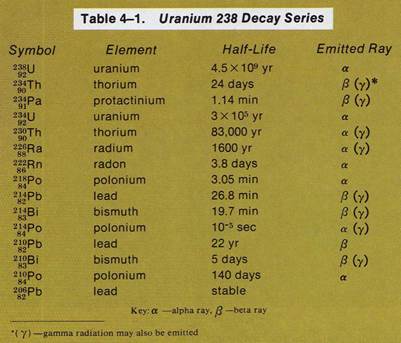
Radioactive elements which can undergo nuclear change are found in Table 4-1. Nine of these elements are- naturally radioactive. Those with atomic numbers greater than uranium (Z= 92) are artificially-made. For example, radioactive radium transmutes into radon, a radioactive gas.
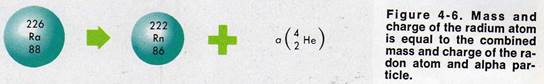 |
A nuclear change may be represented by a nuclear equation. The nuclear equation for the transmutation of radium is
88226Ra → 86222Rn +24He
The word equation for this reaction is :
one atom of radium 226 yields one atom of radon plus one alpha particle
In a nuclear equation, the symbols for the elements are the same as in a chemical equation. In addition, the atomic mass number of each element is written to the top left of the symbol. The atomic number is written to the bottom left of the symbol.
 |
A nuclear equation must be balanced.
It must follow the laws of conservation
of mass and conservation of charge.
The sum of the atomic mass numbers to
the left of the arrow must equal the sum
of the atomic mass numbers to the right
of the arrow. This shows conservation
of mass. Also, the sum of the atomic
numbers (protons) on the left must equal
the sum of the atomic numbers on the
right. This shows conservation of charge.
Inthe radium nuclear decay reaction,
the total atomic mass to the left of the
arrow is 226. This number equals the
sum of the atomic mass numbers to the
right of the arrow (222 + 4 = 226). The atomic number to the left of the arrow is 88. This represents the sum of the atomic numbers to the right of the arrow (86 + 2 = 88).
PROBLEM
1. What symbol in the equation on the previous page is an alpha particle? Why is an alpha particle part of the reaction?
How is a nuclear equation different from a chemical equation?
 A nuclear change is completely different from a chemical or physical change. Ina chemical change, atoms are rearranged. New compounds are formed or elements are separated from compounds. Ina physical change, such as freezing or boiling, a substance changes its physical form. No new elements or compounds are produced. In a nuclear change,one element is changed into a different element. There is a change in the number of protons in the atoms.
A nuclear change is completely different from a chemical or physical change. Ina chemical change, atoms are rearranged. New compounds are formed or elements are separated from compounds. Ina physical change, such as freezing or boiling, a substance changes its physical form. No new elements or compounds are produced. In a nuclear change,one element is changed into a different element. There is a change in the number of protons in the atoms.
Uranium-238 is a radioactive element. It decays through a series of
nuclear reactions to form lead. Lead-206 is a stable isotope. It is
not radioactive. The nuclear reactions through which uranium becomes
lead are known as a nuclear decay series.In each reaction of the
uranium decay series, a different radioactive element is formed.
PROBLEMS
2. Which of the radioactive isotopes in the uranium decay series are
alpha emitters?
3. Which of the radioactive isotopes in the uranium decay series are
beta emitters?
transmutation – перетворення
fission - рощеплення
fusion – злиття (термоядерний синтез)
lead - свинець
nuclear decay series – ряд радіоактивного розпаду