Geothermal Basics Potential Use
Energy
Energy is defined in Webster's dictionary as "capacity for action or performing work." Any action that occurs in nature is accompanied by a reduction in the overall quality of energy while energy is neither destroyed nor created, but transformed into state(s) or form(s) with an overall lower quality or potential. (Matter may be considered as a special from of energy and should also be included in the energy conservation principle stated above when involving nuclear energy.) Higher forms of energy are harnessed by natural or human processes and are downgraded to lower forms of energy and rendered often useless, thus a continuos depletion of useful energy forms occurs in nature.
The various forms of energy include heat, light, microwaves, electricity, potential energy stored in a body held in a force field, say the gravitational field at an elevation, kinetic energy of a moving object, energy associated with the chemical bonds that hold atoms and molecules together and nuclear energy created by transforming matter into energy.
Energy Use.
Energy plays an important role in our lives and its use is a direct measure of the economic well being of a society. The economic health of a country is often measured in terms of the GNP (Gross National Product). Often times, the per capita energy consumption is correlated to the per capita GNP of a country. The following table shows a comparison of the per capita energy consumption of some countries at various levels of economic development and the GNP. The correlation between energy use and per capita GNP, however, does not take into account the energy conservation measures practiced by or instituted in a given country (example, mass transportation), the climatic conditions and the type of economy, that is, whether it is dominated by a service economy, agriculture or industrial production. Note the anomalies between the U. S., Japan and Germany and between Italy and South Korea:
Energy Consumption and Per Capita1,2 and GNP3 (Multiply 106 Btu by 1.0548 to obtain Giga Joules) 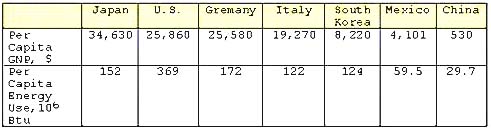
Energy is used in almost every human activity in an industrialized society, examples being:
| 
|
Thus, energy is consumed by us directly as in transportation and household uses as well as indirectly by consuming goods that require energy in their production.
 The U.S. consumes the largest amount of energy per capita on a worldwide basis. Its total annual energy consumption was 90.8 quadrillion Btu2 or 95.8x109 Giga Joules in 1995 which corresponds to a per capita annual consumption of 369 million Btu1,2 or 389 Giga Joules (a barrel of oil or 440 lb of a typical bituminous coal contains 5.6 million Btu or 5.9 Giga Joules). The use of energy in the U. S. is mostly concentrated in large metropolitan areas such as Los Angeles. A major use of energy is in automobiles due to the long distance commuting involved. The annual gasoline consumption for the Los Angeles county alone (excluding aviation) in 1995 was 3.457 billion gallons or 0.458 quadrillion Btu or 0.483x109 Giga Joules.4 Electricity and natural gas delivered to this county in 1996 were 60,498 million KWH and 3,573 million therms5,6 or 377 Giga Joules. The per capita energy consumption of electricity, natural gas and gasoline alone, with a population of 9.488 million for the county, is estimated at 108 million Btu or 114 Giga Joules. Note that this energy consumption is at the end user only and represents only a portion of the total energy required by a population. For example, it does not include the energy required for the production of the electricity by the utility or the energy present in the petroleum that goes into the production of the gasoline by the refinery which are significantly higher, nor does it include the energy required to produce the various goods or consumer items that a population consumes.
The U.S. consumes the largest amount of energy per capita on a worldwide basis. Its total annual energy consumption was 90.8 quadrillion Btu2 or 95.8x109 Giga Joules in 1995 which corresponds to a per capita annual consumption of 369 million Btu1,2 or 389 Giga Joules (a barrel of oil or 440 lb of a typical bituminous coal contains 5.6 million Btu or 5.9 Giga Joules). The use of energy in the U. S. is mostly concentrated in large metropolitan areas such as Los Angeles. A major use of energy is in automobiles due to the long distance commuting involved. The annual gasoline consumption for the Los Angeles county alone (excluding aviation) in 1995 was 3.457 billion gallons or 0.458 quadrillion Btu or 0.483x109 Giga Joules.4 Electricity and natural gas delivered to this county in 1996 were 60,498 million KWH and 3,573 million therms5,6 or 377 Giga Joules. The per capita energy consumption of electricity, natural gas and gasoline alone, with a population of 9.488 million for the county, is estimated at 108 million Btu or 114 Giga Joules. Note that this energy consumption is at the end user only and represents only a portion of the total energy required by a population. For example, it does not include the energy required for the production of the electricity by the utility or the energy present in the petroleum that goes into the production of the gasoline by the refinery which are significantly higher, nor does it include the energy required to produce the various goods or consumer items that a population consumes.
Energy Sources. Primary sources of energy are fossil fuels such as natural gas, oil, coal and to a lesser extent, nuclear by fission of radioactive elements (in France however, majority of electricity is produced by nuclear), solar in direct heating, in photovoltaic cells, rivers that provide hydroelectricity which in certain regions such as the U. S. Pacific Northwest and in Norway and Sweden is very significant (note that it is the solar energy that actually "drives" the rivers), wind for turning turbines to generate electricity, geothermal, biomass and possibly oil shale in the future.
Energy Conversion. In most current applications, the energy contained in fossil fuels and biomass as the chemical bond energy is harnessed by combustion. These combustion based sources provide as much as 90% of the current energy supply in the U.S. The combustion process, however, produces pollutants and conversion efficiency of the fuel bound energy to electricity with the intermediate step involving combustion is limited by the temperature at which the heat may be utilized. A more efficient and environmentally responsible way of converting this fuel bound energy consists of utilizing fuel cells which convert the fuel bound energy directly into electricity. Fuel cells, however require clean fuels such as natural gas. Dirty fuels such as coal and biomass may be converted to clean fuels by partial combustion or gasification followed by cleaning the gas thus derived, and then either fueling a fuel cell or a gas turbine with the clean gas.
Energy Reserves and Trends1,2. The U.S. has large reserves of coal and supplies both the domestic and the export markets. Coal was the principal source of energy in the U.S. and its use peaked in the 1930s and since then its consumption has been relatively flat with some minor peaks and valleys. Large scale production of oil mostly brought about by the advent of the automobile was responsible for the decline in the steady growth of coal usage. Nuclear energy took off in the late 1950s to early 1960s but its usage flattened off in the 1960s to 1970s with only 10% of the total U.S. energy demand being met by it. In contrast, France meets essentially all its energy demands for generating electricity by Nuclear. Natural gas usage took off in the U.S. in the 1920s and provides a clean burning energy source compared to the other fossil fuels. Finally, solar energy whose use was encouraged in the late 1970s by President Carter never really took off, the monetary incentives for encouraging its use and the research grants were not given the necessary support by subsequent administrations.
Energy use over the next two decades is expected to increase significantly throughout the world, with highest growth rates in Asia. By the year 2015 world energy demand is projected to be around 562 quadrillion Btu or 593x109 Giga Joules. This growth represents more than a 50% increase over the consumption in 1995. Two-thirds of this increase in energy consumption is expected to be due to the developing countries concentrated mostly in Asia where energy growth is projected to be on an average of 4.2% annually, while for industrialized economies it is projected to be 1.3%.
Other than nuclear power, all sources of energy are projected to grow: Oil use is expected to exceed 100 million barrels per day by 2015 which is a growth of 50% over 1995. Coal use is projected to be in excess of 7.3 billion ST or 6.6 billion MT or Mega Grams by 2015 on a world wide basis, compared to 5.1 billion ST or 4.6 billion MT or Mega Grams in 1995. Natural gas usage is expected to increase at 3.1% annually and by 2015 and is projected to be the principal fossil fuel for the world.
Due to these tremendous increases in fossil fuel usage world wide, the carbon emissions to the atmosphere are expected to increase by about 60% by 2015 over the 1990 level.
Energy Use and the Environment. A conflict, thus arises between use of energy and the environment, this conflict brought about for the first time in the life of our planet, in the 20th century by the large scale usage of energy. The harnessing of the energy contained within a fuel by combustion produces pollutants such as oxides of sulfur, oxides of nitrogen and unburned hydrocarbons which are introduced into the atmosphere, the amount depending on the degree of pollution abatement measures incorporated. All fossil fuels also introduce the greenhouse gas carbon dioxide into the atmosphere while nuclear energy produces radioactive waste. In the past, the governing laws for engineering design in the industry had been the application of mass, momentum and energy equations but in the future the environmental impact will be included more and more as one of the laws in the design work.
The importance of minimizing the adverse effects on the environment or what is emitted to the atmosphere may be appreciated by realizing that the earth's surface is covered by a precious layer of air that is only 10 miles or 16 kilo meters in thickness (the troposphere) which is quite thin when compared to the earth's diameter of 8000 miles or 12,800 kilo meters.
Quiz
1. Which of the following energy conversions produces the least amount of pollution?
a) Fossil Fuels
b) Fuel Cells
c) Coal
d) Biomass
2. Which of these produce energy?
a) hot water
b) automobile
c) wind
d) all the above
3. Which country or countries consumes the largest amount of energy per capita?
a) Japan
b) United States
c) Russia
d) Germany
e) The entire continent of Africa
4. Which of the three fossil fuels (Natural Gas, Coal, & Oil) is the cleanest fuel and simplest in terms of composition?
a) Natural Gas
b) Coal
c) Oil
5. Dinosaur bones can be used as fuel.
a) true
b) false
c) don't know
6. Petroleum is found
a) on the surface of Mars.
b) in whales.
c) in sedimentary rock formations underground.
b) in the fuel tank of the DeLorean in the movie "Back to the Future".
7. Petroleum is used
a) only sparingly because it is only available in remote areas.
b) very often for vehicles and power plants.
c) in beers and wine as a key ingredient.
| Environmental Impacts The earth is being stressed by the large amounts of pollutants being introduced into the atmosphere. Example are: At the urban level, photochemical oxidants are causing smog (the first recording of aerosols was made in 1943 and brought to the public attention in the Los Angeles Times). In the troposphere, acid rain is being formed and the greenhouse effect increasing (the first presentation of global warming was made in the newspapers in the mid 1980s). In the stratosphere, the ozone which is tri-atomic oxygen (O3) is being depleted (DuPont had to stop making chloro fluoro carbons or CFCs which play a role in the depletion of the O3; the Nobel prize was awarded to the discoverer of this phenomena). Urban Level. The automobile is a major source of pollution in the urban areas and is responsible for the vast majority of the primary pollutants such as NO, hydrocarbons (HCs) and CO. This is strongly supported by data collected since 1940 and it can be seen that a decline in each of these pollutant concentrations was observed as legislatures for control were implemented. The NO is oxidized to NO2 which is a brown gas and causes the brown haze observed in the early morning period. In the south coast basin, half of the NOx is produced from automobiles. Later in the morning under the action of sunlight, NO2 decomposes generating NO and O. The atomic O combines with O2 to produce the secondary pollutant O3. The NO can react with the HCs forming PAN, aerosols etc. The automobiles (and not refineries) were first identified as the major sources of HCs in 1950, and in 1963 the first control was implemented to keep the HCs from escaping from its crankcase. The first control of the automobile exhaust was implemented in 1966 to reduce the HCs and CO emissions and finally, in 1970 the Clean Air Act was passed largely due to Edmund Muskie's efforts. This paved the path for the establishment of the EPA by Nixon. In 1971, the second exhaust control was passed to limit the NOx emissions. Further reductions (by 90%) in the HCs/CO and NOx were enacted in 1975 and 1977, respectively. In 1966, the NOx increased when the HC emissions control was implemented. In 1973, it was determined that the implementation of exhaust gas recycle to limit the NOx increased the HC emissions. Thus, the NOx and HCs emissions have shown an inverse relationship. So far, the use of automobiles in the urban areas has been unscabed while the control strategies have been essentially exhausted. For example, currently (in 1997), the CO emissions which are essentially all from cars with control devices in the Los Angeles south coast basin are as high as 8,000 ST/D or 7260 MT/D or Mega Grams/D (CO replaces O2 in the blood and starves the body/brain from O2). Thus, measures are being instituted in the Los Angeles are such as diamond lanes, car pools, increased parking fee and encouragement of electric cars (combination gasoline/electric cars have been also announced, equipped with gas turbines or diesel engines, the fuel cell being ideal, however, because of its significantly higher efficiency). Troposphere. The lower region of the atmosphere which is characterized by decreasing air temperatures as the altitude is increased is the troposphere. Types of pollution within the troposphere include those caused by (1) SO2 as the primary pollutant with particulates as the secondary pollutant formed from the SO2 (the well known London Killer Fog episode that occurred in the mid 20th. century being an example), and (2) NOx and organics as the primary pollutants with O3, HNO3, H2SO4, particulates and Peroxy Acetyl Nitrate (PAN) as the secondary pollutants formed by photochemical reactions from the primary pollutants (the air pollution in Los Angeles being an example of this type of pollution). In the troposphere SOx and NOx produce their respective acids and cause the acid rain. Also the build up of CO2 and O3 in this region of the atmosphere causes the greenhouse effect (CO2 is transparent to ultra violet radiation that strike the earth's surface during the daytime but CO2 is not transparent to infra red which is reradiated by the earth's surface). Taxes are being levied on the airlines when flying over some of the European countries (e.g.. Norway) to force a reduction in these emissions, leading both Lufthansa and Swiss Air to push GE in designing jet engines with reduced NOx emissions. A brief overview of the role played by O3 and associated species, as well as the predominant role played by the hydroxyl (OH) radical in tropospheric chemistry is presented in the following: Photochemical Air Pollution. Under the action of sunlight (essentially in the visible region of the spectrum, noting that majority of the ultra violet radiation from the sun is blocked by the O3 present in the upper regions of earth's atmosphere), photochemical reactions occur which convert the primary pollutants such as Volatile Organics Compounds (VOCs), NO and NO2 with the ultimate formation of the secondary pollutants such as O3, PAN, HNO3, particulates (such as H2SO4, sulfates, nitrates, organics). VOCs. VOCs are responsible for the formation of a number of secondary pollutants such as PAN and particulates and are also responsible for the formation of a major portion of the O3 where the NO concentration is low, such as in less polluted rural areas. OH radical. The chemistry of the atmosphere is dominated by the reactive OH radical in the daytime (while the nitrate NO3 radical plays a major role in the night time), resulting in photochemical air pollution, acid rain and fogs, and the toxic organics. A major source of the OH radical is the O3 which under the action of light decomposes to generate the atomic O which in turn reacts with H2O to produce the OH radical. Photolysis of nitrous acid which is formed from NO2 also produces the OH radical. Halogen Atoms. Chlorine atoms play a similar role in the chemistry of the stratosphere as the reactive OH radical and oxidize most organic compounds with high reaction speeds. A mechanism proposed for the production of Cl atoms involves the reaction of N2O5 with sea-salt aerosol. Ozone Control Strategies and Health Issues. The amount of O3 formed depends on the VOC/NOx ratio of the air mass and is the greatest at smaller VOC/NOx ratios. The reactivity of each of the VOCs is also different and thus, O3 control strategies for highly polluted urban regions should be implemented in conjunction with stringent NOx control policy while taking into account the reactivity of the individual VOCs. The emissions for compressed natural gas fueled vehicles show very low concentrations of the more reactive VOCs and thus, this fuel should be an attractive alternative. Finally, OH, NO3 and O3 play a major role in the formation and fate of airborne toxic chemicals, mutagenic polycyclic aromatic hydrocarbons and fine particulates (PM10 and PM2.5). Epidemiological studies have shown a direct link between mortality and particles less than 10 microns. Stratosphere. The region of the atmosphere above the troposphere in which the temperature starts increasing with altitude is the stratosphere and there is interaction and mixing between the stratosphere and the troposphere. The stratosphere contains large concentration of O3 which is responsible for the increase in temperature of the stratosphere. The O3 is formed here by the Chapman cycle which is the steady state formation and destruction of O3. The atomic O formed by absorption of ultra violet radiation (<220 nm) by the O2 reacts with O2 to form O3 (the presence of a third body to take away the energy from the newly formed O3 is also required). O3 is toxic to breathe but in the upper layers of the atmosphere O3 blocks the ultra violet radiation. With the addition of destruction cycles, however, this steady state is destroyed and O3 is depleted. Perturbations in the O3 level may be caused by CFCs and possibly the proposed high speed civil transport (HSCT) plane. Ultra violet radiation can cause cataracts, skin ailments and crop damage. 90 to 95% of the O3 has been destroyed in the South Pole as measured in 1993 (it should be noted that the O3 measurement is also effected by solar intensity and seasons). The NO introduced into this zone reacts with the O3 and thus, the protective O3 layer is depleted. Thus, the development of the HSCT which will fly in the troposphere and is NASA's highest priority (in order to maintain U.S.'s leadership role in the aircraft design area, help the balance of payments and the U.S. industry), is at stake. The plane which would be capable of carrying 300 passengers and traveling at Mach 2.4 is technically feasible but is unable to meet the NOx goals at the present time. Alternatives. Alternatives available are: (1) Conservation - one way to enforce this is to add an energy tax (Japan uses 1/3 of the energy per capita as the U.S. because of higher energy costs in Japan), (2) more efficient energy systems such as cogeneration and fuel cells (with fuel cells the efficiency may be almost doubled resulting in a 50% reduction in fuel use, (3) these systems still produce CO2, pointing towards use of solar energy, and finally (4) evolution of an electric society which has already started with the introduction of the electric automobiles. |
Fuel Cell
The following provides some background information on fuel cells in general followed by a descriptions of the characteristics of fuel cells by major type. An excellent in-depth treatment of the subject may be found in the U. S. Department of Energy publication titled "Fuel Cells - A Handbook" by Hirschenhofer et. al., January 1994.
Fuel cells as energy conversion devices have a number of advantages; some of these advantages being: · high energy conversion efficiency which is relatively independent of size · good part load characteristics · modular design and flexibility of size · low environmental impact · rejection of heat at high temperature in some of the fuel cell types, which is suitable for cogeneration · citing ability due to the favorable environmental signature · quick response to load changes.
Fuel cells, however do have certain disadvantages which are listed below: · sensitivity to certain contaminants that may be present in the fuel such as sulfur and chlorides · current capital costs on a $/KW basis are high · lack of the field data on endurance/reliability.
One of the main advantages of fuel cells is the high conversion efficiency which may range from 40 to 60% based on lower heating value (LHV) of the fuel. The fuel conversion efficiency is higher than that of most energy conversion systems, the efficiency advantage becoming more significant at the smaller scales since the efficiency of fuel cells is nearly constant with size. Furthermore, the heat rejected from some of the fuel cell types such as the Molten Carbonate and the Solid Oxide fuel cells is at high temperature and thus, is available for cogeneration applications. Thus, fuel cell plants can be constructed in a wide range of electrical output, from less than a KW to sizes in excess of a MW. Fuel cells produce virtually no gaseous, solid or noise emissions except unless turbomachinery is included in the plant.
Fuel cells are sensitive to certain fuel contaminants such as sulfur and chlorides. Furthermore, the fuel entering the cell has to be gaseous and in some cases has to be hydrogen which leads to the requirement of a fuel pre-processor such as a reformer. In some fuel cell designs, however, the reformer has been incorporated within the fuel cell itself.
Thus, fuel cells can be classified by whether the fuel is processed outside (externally reformed) or inside (internally reformed), by the type of electrolyte or by the temperature of operation. A brief description of the major types of fuel cells characterized by the type of electrolyte employed is presented in the following.
Trends
The world is running out of cheap energy such as oil and natural gas that allowed rapid industrialization in some nations, and its capacity to withstand the environmental impact of burning coal as a substitute. Moving to a more expensive and less polluting energy source is required and the related issues need to be resolved globally because these issues affect everyone. In the past, industrialized societies were more concerned with insufficient supply of energy rather than its cost. However, with the sudden increase in oil prices in the '70s, the emphasis changed while the environmental and sociopolitical costs of energy came to be recognized also in the '70s, resulting in energy seeming to be costlier from both supply and use sides.
It is uncertain whether this trend will sustain itself in the '90s, but its importance can be realized by considering the large growth in world's population and its per capita energy use. For example, if the oil and gas consumption are allowed to grow unchecked, within 30 to 40 years 80% of these resources will be depleted. Coal, solar and nuclear energy are more abundant but require more involved and expensive conversions into electricity or liquid fuels.
There are both external and internal costs brought about by the finiteness of the earth's capacity to withstand the environmental impacts. External costs are associated with environmental disruptions on society but are not reflected by the monetary values assigned to energy. Internal costs are costs due to measures such as pollution control equipment to reduce the external costs. External costs have been rising despite a substantial increase in the internal costs (e.g.. in the U.S. the costs of supplying petroleum have increased by 25% while costs of generating electricity have risen by 40% over the past 20 years). The external costs are difficult to quantify but civilization depends heavily on regulating water supply, controlling pests and pathogens and maintaining a tolerable climate. Climate governs most of environmental processes on which our well-being depends and global climatic changes brought about by greenhouse gases could be devastating. The other major danger to public health is particulates from the SO2 emissions. Fixing these external costs for coal will add about 30% to the cost of energy.
The CO2 problem may be postponed by switching to natural gas but at huge costs. Nuclear energy is less disruptive on the climate but requires development of much safer reactors and management of nuclear wastes. Solar energy is clearly the long-term solution from a standpoint of minimizing the external costs but at the present time remains too expensive. The near term solution is to transition into low impact energy supply technologies (without disrupting economic growth) and increase end use efficiency. If the energy consumption in the developing countries increases to near that of the developed countries per capita, CO2 and other pollutants would effect both locally and globally on a scale much larger than ever experienced. Climate change could have profound disruptions especially for the Southern Hemisphere nations which would also effect the Northern Hemisphere nations since we are all connected by trade etc. Thus, international cooperation in energy research in the areas of long-term alternatives is crucial.
Biomass Energy Basics
Sources of Biomass
We have used biomass energy or "bioenergy"—the energy from plants and plant-derived materials—since people began burning wood to cook food and keep warm. Wood is still the largest biomass energy resource today, but other sources of biomass can also be used. These include food crops, grassy and woody plants, residues from agriculture or forestry, and the organic component of municipal and industrial wastes. Even the fumes from landfills (which are methane, a natural gas) can be used as a biomass energy source.
Benefits of Using Biomass
Biomass can be used for fuels, power production, and products that would otherwise be made from fossil fuels. In such scenarios, biomass can provide an array of benefits. For example:
- The use of biomass energy has the potential to greatly reduce greenhouse gas emissions. Burning biomass releases about the same amount of carbon dioxide as burning fossil fuels. However, fossil fuels release carbon dioxide captured by photosynthesis millions of years ago—an essentially "new" greenhouse gas. Biomass, on the other hand, releases carbon dioxide that is largely balanced by the carbon dioxide captured in its own growth (depending how much energy was used to grow, harvest, and process the fuel).
- The use of biomass can reduce dependence on foreign oil because biofuels are the only renewable liquid transportation fuels available.
- Biomass energy supports U.S. agricultural and forest-product industries. The main biomass feedstocks for power are paper mill residue, lumber mill scrap, and municipal waste. For biomass fuels, the feedstocks are corn (for ethanol) and soybeans (for biodiesel), both surplus crops. In the near future—and with NREL-developed technology—agricultural residues such as corn stover (the stalks, leaves, and husks of the plant) and wheat straw will also be used. Long-term plans include growing and using dedicated energy crops, such as fast-growing trees and grasses, that can grow sustainably on land that will not support intensive food crops.
Geothermal Energy Basics
The Earth's heat—called geothermal energy—escapes as steam at a hot springs in Nevada.
Many technologies have been developed to take advantage of geothermal energy—the heat from the earth. This heat can be drawn from several sources: hot water or steam reservoirs deep in the earth that are accessed by drilling; geothermal reservoirs located near the earth's surface, mostly located in western states, Alaska, and Hawaii; and the shallow ground near the Earth's surface that maintains a relatively constant temperature of 50°-60° F.
This variety of geothermal resources allows them to be used on both large and small scales. A utility can use the hot water and steam from reservoirs to drive generators and produce electricity for its customers. Other applications apply the heat produced from geothermal directly to various uses in buildings, roads, agriculture, and industrial plants. Still others use the heat directly from the ground to provide heating and cooling in homes and other buildings.
Other geothermal resources exist miles beneath the earth's surface in the hot rock and magma there. In the future, these resources may also be useful as sources of heat and energy.
Solar Energy Basics
The sun's heat and light provide an abundant source of energy that can be harnessed in many ways. There are a variety of technologies that have been developed to take advantage of solar energy. These include concentrating solar power systems, passive solar heating and daylighting, photovoltaic systems, solar hot water, and solar process heat and space heating and cooling.
Solar power can be used in both large-scale applications and in smaller systems for the home. Businesses and industry can diversify their energy sources, improve efficiency, and save money by choosing solar technologies for heating and cooling, industrial processes, electricity, and water heating. Homeowners can also use solar technologies for heating and cooling and water heating, and may even be able to produce enough electricity to operate "off-grid" or to sell the extra electricity to the utilities, depending on local programs. The use of passive solar heating and daylighting design strategies can help both homes and commercial buildings operate more efficiently and make them more pleasant and comfortable places in which to live and work.
Beyond these localized uses of solar power, utilities and power plants are also taking advantage of the sun's abundant energy resource and offering the benefits to their customers. Concentrating solar power systems allow power plants to produce electricity from the sun on a larger scale, which in turn allows consumers to take advantage of solar power without making the investment in personal solar technology systems.
Solar power technologies, from individual home systems to large-scale concentrating solar power systems, have the potential to help meet growing energy needs and provide diversity and reliability in energy supplies.
Wind Energy Basics
We have been harnessing the wind's energy for hundreds of years. From old Holland to farms in the United States, windmills have been used for pumping water or grinding grain. Today, the windmill's modern equivalent—a wind turbine—can use the wind's energy to generate electricity.
Wind turbines, like windmills, are mounted on a tower to capture the most energy. At 100 feet (30 meters) or more aboveground, they can take advantage of the faster and less turbulent wind. Turbines catch the wind's energy with their propeller-like blades. Usually, two or three blades are mounted on a shaft to form a rotor.
A blade acts much like an airplane wing. When the wind blows, a pocket of low-pressure air forms on the downwind side of the blade. The low-pressure air pocket then pulls the blade toward it, causing the rotor to turn. This is called lift. The force of the lift is actually much stronger than the wind's force against the front side of the blade, which is called drag. The combination of lift and drag causes the rotor to spin like a propeller, and the turning shaft spins a generator to make electricity.
Wind turbines can be used as stand-alone applications, or they can be connected to a utility power grid or even combined with a photovoltaic (solar cell) system. For utility-scale sources of wind energy, a large number of wind turbines are usually built close together to form a wind plant. Several electricity providers today use wind plants to supply power to their customers.
Stand-alone wind turbines are typically used for water pumping or communications. However, homeowners, farmers, and ranchers in windy areas can also use wind turbines as a way to cut their electric bills.
Small wind systems also have potential as distributed energy resources. Distributed energy resources refer to a variety of small, modular power-generating technologies that can be combined to improve the operation of the electricity delivery system.
Alternative Fuels
When you drive an alternative fuel or flexible fuel vehicle, you don't have to rely entirely on petroleum as a fuel. You can use an alternative fuel designed for the vehicle. The alternative fuel will help reduce our dependency on imported petroleum and produce fewer harmful tailpipe emissions.
Alternative fuels include:
- Biofuels
- Gaseous fuels
- Gas-to-liquid fuels
- Electricity
Biofuels
Biofuels are made from biomass. Today, biofuels used in vehicles are commonly blended with petroleum. For example, E85 is 85% ethanol and 15% petroleum. And B20—a biodiesel blend—is 20% biofuels and 80% diesel. Pure biodiesel (B100) can be used, but it currently requires engine modifications and may not be suitable for wintertime use.
Gaseous Fuels
Gaseous fuels aren't in liquid form. They're "gaseous" as their name suggests. Gaseous fuels include natural gas and hydrogen, as well as hydrogen-natural gas fuel blends.
There are two types of natural gas: compressed natural gas (CNG) and liquefied natural gas (LNG). Dedicated natural gas vehicles run only on natural gas. Meanwhile, bi-fuel natural gas vehicles have two separate fueling systems, which enable them to use either natural gas or petroleum.
Unlike natural gas, hydrogen is still evolving as a transportation fuel. Hydrogen has been used effectively in a number of internal combustion engine vehicles as pure hydrogen mixed with natural gas. But hydrogen fuel-cell vehicles aren't yet commercially available.
Gas-to-Liquid Fuels
Gaseous fuels can be converted into liquid fuels, which can be refined into gasoline and diesel. These gas-to-liquid fuels are also known as Fischer-Tropsch fuels.
Electricity
Electricity can be used as a transportation fuel. It's used to power batteries in electric and plug-in hybrid electric vehicles. And fuel cell vehicles use electricity produced from an electrochemical reaction that takes place when hydrogen and oxygen are combined in the fuel cell "stack."
| Fuel Definitions |
Key Concepts
A fuel is a substance that releases usable energy either through:
|
Geothermal Basics Potential Use
· 3.1. What is the official government estimate of potential geothermal electric resources in the U.S.?
· 3.2. Are there other examples of how geothermal resources are utilized?
· 3.3. How much energy is geothermal electricity capable of supplying to the U.S?
· 3.4. Where are geothermal resources located?
· 3.5. "How much electricity can geothermal supply worldwide?"