Preparing of a semiemulsion.
Materials:
1) 0.5% solution of polyacrylamide in fresh water;
2) Water soluble surfantant – NEONOL AF9-12
3) Diesel fuel
Measure out with the cylinder 38 ml of 0.5% solution of polyacrylamide prepared in fresh water and pour it out into the porcelain mug. With a minimum rate of mixing (to avoid foaming), using the syringe, inject 2 ml of NEONOL AF9-12 into the polyacrylamide solution, and then after its dissolving, inject a hydrocarbon fluid – 160 ml (80% vol) diesel fuel into the solution. Follow the techniqul description (see point 1 and Notes) for obtaining a high-disperse emulsion. Diesel fuel is tj be injected by portions: a quarter of a total amount with a minimum rate of mixing and the remaining ¾ of the total amount – faster, approximately within 2-3 minutes. After the injection of the total amount of the diesel fuel it is necessary to continue mixing for 10 minutes more, and then it is possible to start the emulsion analysis with the help of microscope. Sketch a pattern obtained.
The emulsions obtained in this way can be used as high-efficient fluids for hydraulic fracturing of formation, since they have a low friction pressure loss and they are stable at the reservoir temperatures of 60-80 C.
Notes:
There are two opposite processes occurring during the reaction of disperse phase emulsification – dispersion and coalescence. It follows from the analysis of emulsification process that the more energy is spent during the emulsion formation, the more high-disperse system is formed.
When obtaining the emulsion it is reasonable to use as much as possible the physicochemical properties of the surfactant and a specific character of viscosity level increasing. Thus, during the period of adding a disperse phase into the surfactant solution, surface tension at the interface of the mixed fluids is minimal, since the concentration of surfactant is maximal. During this period the viscosity of the system is also minimal due to the low content of disperse phase. These two factors contribute to efficient mixing of the fluids, which are insoluble in each other, thus maximum dispersion of the injected fluid occurs and the smallest drops of dispersed phase are obtained, and high mixing rate prevents the coalescence of the drops obtained. With the subsequent injection of a disperse phase into the system, the extension of drops obtained occurs, as surfactant concentration in the system decreases. Thus viscosity of emulsion increases, and, on the one hand, this prevents from efficient mixing and crushing of the drops, but, on the other hand, this prevents from coalescence. Therefore, in the process of emulsification some (smaller) portion of disperse phase has to be injected slowly as a thin stream with intensive mixing. It is necessary for maximum drop crushing. The subsequent portions can be injected faster. If the dispersed phase is injected too fast, even continuous mixing at high speed will not allow obtaining a high-dispersed emulsion.
Part 2. Determination of dispersion with a microscope.
Dispersion is determined by its visual observation under a microscope. For a quantitative assessment it is needed to make straight measurements of dispersed particles (globules) size. From the big amount of measurements integral curves of globules distribution by size and by value of a total surface area are to be statistically obtained.
The main equations for calculation of integral distribution curves are:
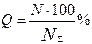 (1),
(1),
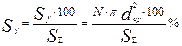 (2),
(2),
where Q and Sy are relative sizes of the globules and their relative specific surface area within the given fraction with the upper and lower limits
N and Sf are the numbers of globules of the fraction and their total surface area with an average diameter
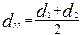
N∑ and S∑ are the total number of the measured globules and their averaged total surface area.
Equipment
1) microscope (resolution x250)
2) slide
3) microglasses
The drop of the emulsion is to be put onto the slide and covered on by a microglass. The size of emulsion’s globules is to be determined with the help of microscope. Then determine the total number of globules per unit surface and the number of the globules by fractions. According to formulas 1 and 2 determine the relative size of the globules and the total surface area of fraction with the average diameter. The measurement results are to be recorded in table 1.
The data in table 1 is to be presented graphically as distribution curves (the vertical axis is relative size Q and relative specific surface area of fraction Sy (%), and the horizontal axis is average globules diameter).
Table 1
Size of globules
 ¸ ¸  , µ (microns) , µ (microns)
| Number of globules at the fraction, N | Total surface area Sf, µ2 | Relative size of globules, Q, % | Relative specific surface area of fraction, Sу, % |
| 1-5 | ||||
| 6-10 | ||||
| 11-15 | ||||
| 16-20 | ||||
| Etc. | ||||
| NS | SS |
Questions for self-testing:
1. Describe the main principles of emulsion classification by the type of disperse phase and dispersion medium, by the content of disperse phase, by the level of dispersion and by the level of interphase interaction.
2. Explain how you can regulate the processes of coalescence and dispersion of globules while preparing an emulsion.
3. Name the factors which can effect the value of interfacial tension and the viscosity of emulsions.
4. Explain the reasons for dispersing oil in water duringat alkaline and acid treatments.
5. Describe the areas of practical application of direct, inverse, multiple emulsions and semiemulsions in the oil industry.